Enzymes are unique proteins that act as catalysts in biological reactions. They play a crucial role in speeding up chemical reactions within cells without being consumed or altered in the process. Enzymes are involved in various metabolic pathways and are essential for the proper functioning of biological systems.
A defining feature of enzymes is their ability to reduce the activation energy needed for a chemical reaction to take place. Activation energy is the energy barrier that must be overcome for a chemical reaction to start. Enzymes facilitate this process by providing an alternative pathway with lower energy requirements, allowing reactions to proceed more rapidly.
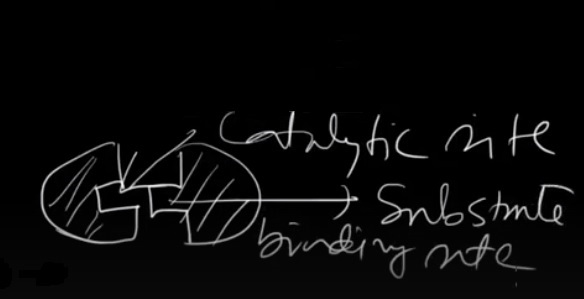
Enzymes are highly specific in their actions, meaning that each enzyme has a particular substrate or group of substrates it can bind to and catalyze. The specific area on an enzyme where the substrate attaches is called the active site. The interaction between the enzyme and its substrate is often described as a "lock and key" mechanism, where the substrate fits precisely into the active site of the enzyme.
Enzyme activity can be influenced by various factors, including temperature, pH, substrate concentration, and the presence of inhibitors or activators. Each enzyme has an optimal range of conditions in which it functions most efficiently.
Enzymes have diverse roles in living organisms, such as facilitating digestion, producing energy, synthesizing molecules, and removing waste products. In the absence of enzymes, numerous vital biochemical reactions would proceed too slowly to support life.
Structure of Enzyme:
The structure of enzymes is intricately organized to carry out their catalytic functions. Enzymes are commonly formed by lengthy sequences of amino acids that intricately fold into precise three-dimensional configurations. This folding is critical for creating the unique structure of each enzyme.
The primary structure of an enzyme refers to the linear sequence of amino acids in the protein chain. This sequence is determined by the DNA sequence of the gene that encodes the enzyme. The exact sequence of amino acids determines how the enzyme folds and shapes its overall structure.
The secondary structure of enzymes arises from interactions between neighboring amino acids in the chain. The two common secondary structures found in enzymes are alpha helices and beta sheets. These structures arise from hydrogen bonding between the amino acid backbone atoms.
Tertiary structure refers to the overall three-dimensional shape of the enzyme. It is determined by interactions between distant amino acids in the protein chain. These interactions include hydrogen bonding, ionic interactions, disulfide bridges, and hydrophobic interactions. The tertiary structure of an enzyme is crucial for its catalytic activity.
Certain enzymes are made up of just one polypeptide chain, whereas others consist of multiple chains. The way these chains are organized and interact with each other forms the enzyme's quaternary structure. The quaternary structure is only present in enzymes with multiple subunits. It influences the stability, activity, and regulation of the enzyme.
The active site of an enzyme is the specific region within its structure where the substrate attaches and the chemical reaction is carried out. The active site is typically a small, well-defined crevice or pocket within the enzyme's structure. The active site possesses an exceptional structure that perfectly matches the contour of the substrate. This selectivity enables the enzyme to precisely recognize and bind to its substrate, facilitating the catalytic reaction.
The overall structure of enzymes is critical for their function. Any alteration in the enzyme's structure—like denaturation due to high temperatures or extreme pH—can interfere with its function and reduce or halt its activity. Proper folding and maintenance of the enzyme's structure are essential for its stability and functionality.
Classification of Enzyme:
Enzymes are grouped into different categories depending on the kind of reaction they facilitate. Here are the primary classes of enzymes:
Oxidoreductases: These enzymes facilitate oxidation-reduction reactions by transferring electrons or hydrogen atoms between molecules. Examples include dehydrogenases and oxidases.
Transferases: Transferases move functional groups like methyl, phosphate, or glycosyl groups from one molecule to another. Kinases and transaminases are examples of transferases.
Hydrolases: Hydrolases are enzymes that catalyze hydrolysis reactions, breaking chemical bonds by adding water molecules. Examples include lipases, proteases, and carbohydrases.
Lyases: Lyases catalyze reactions that involve the removal or addition of groups to double bonds, resulting in the formation of new double bonds or the creation of new rings. Decarboxylases and synthases are examples of lyases.
Isomerases: Isomerases are enzymes that facilitate the rearrangement of atoms within a molecule, transforming it into its isomer form. Examples include racemases and mutases.
Ligases: Ligases participate in joining two molecules together, using ATP as an energy source. DNA ligase is an example of a ligase enzyme.
These classes represent a broad categorization of enzymes based on their functions. Each enzyme class plays a specific role in various metabolic pathways, contributing to the overall functioning of living organisms.
Here are some examples of enzymes:
Amylase: Amylase is an enzyme that breaks down starch into simpler sugars like maltose and glucose through hydrolysis. It is found in saliva and pancreatic secretions and plays a role in the digestion of carbohydrates.
Protease: Proteases are enzymes that break down proteins by hydrolyzing peptide bonds. Examples include pepsin, trypsin, and chymotrypsin. Pepsin is found in the stomach and helps in the initial digestion of proteins.
Lipase: Lipases are enzymes that catalyze the hydrolysis of lipids (fats) into fatty acids and glycerol. They are secreted by the pancreas and aid in breaking down and absorbing fats from the diet.
Catalase: Catalase is an enzyme found in almost all living organisms. It acts as a catalyst for the decomposition of hydrogen peroxide into water and oxygen. Catalase plays a vital role in protecting cells from oxidative damage caused by reactive oxygen species.
DNA Polymerase: DNA polymerase is a vital enzyme that plays a crucial role in the process of DNA replication. It catalyzes the addition of nucleotides to the growing DNA strand during replication. DNA polymerase ensures accurate copying of the DNA molecule.
RNA Polymerase: RNA polymerase is the enzymatic catalyst accountable for the synthesis of RNA molecules using a DNA template in a process known as transcription. It catalyzes the formation of RNA molecules based on the DNA sequence.
ATP Synthase: ATP synthase is an enzyme found in the inner membrane of mitochondria. It is essential in cellular respiration, facilitating the production of ATP (adenosine triphosphate) from ADP (adenosine diphosphate) and inorganic phosphate. ATP synthase is also present in chloroplasts during photosynthesis.
Mechanism of Enzyme Reaction:
The mechanism of enzyme reactions involves a series of steps that allow enzymes to catalyze biochemical reactions. Here’s a basic explanation of how enzymes work:
Substrate Binding: The enzyme and its specific substrate(s) come together at the active site of the enzyme. The active site is a region of the enzyme that complements the shape and chemical properties of the substrate. This interaction is commonly compared to a "lock and key" model, where the substrate fits precisely into the enzyme's active site.
Enzyme-Substrate Complex Formation: Once the substrate attaches to the enzyme’s active site, an enzyme-substrate complex is formed. This complex helps position the substrate accurately, allowing the chemical reaction to proceed efficiently.
Catalysis: The enzyme aids in transforming the substrate into product(s) by reducing the amount of activation energy needed for the reaction to take place. The active site provides an environment that stabilizes the transition state, which is a high-energy state that the substrate must pass through to form the product(s).
Product Formation and Release: The enzyme catalyzes the conversion of the substrate into the product(s). The reaction may involve bond formation, bond cleavage, or other chemical transformations. Once the reaction is complete, the product(s) are released from the active site of the enzyme.
Enzyme Regeneration: After releasing the product(s), the enzyme returns to its original form and can catalyze another round of the reaction with additional substrate molecules. The enzyme is not consumed in the reaction and can continue to function repeatedly.
Main factors that affect enzyme activity:
Temperature: Enzymes have an optimal temperature at which they function most efficiently. As the temperature rises, enzyme activity typically increases because molecules move faster and collide more frequently. However, excessively high temperatures can denature the enzyme, altering its structure and rendering it nonfunctional.
pH Level: Enzymes also have an optimal pH at which they exhibit maximum activity. pH influences the enzyme’s charge and shape, which can affect how well it binds to the substrate and carries out the reaction. Deviations from the optimal pH can disrupt enzyme activity and lead to denaturation.
Substrate Concentration: Enzyme activity is influenced by the concentration of the substrate. Initially, as substrate concentration increases, enzyme activity also increases as more substrate molecules are available for binding to the enzyme's active sites. However, beyond a certain point, the enzyme becomes saturated with substrate, and further increases in substrate concentration do not significantly affect the rate of the reaction.
Enzyme Concentration: The concentration of enzymes directly affects the rate of the reaction. As the enzyme concentration increases, more active sites are available for substrate binding, resulting in higher catalytic activity. However, at a certain point, further increases in enzyme concentration do not lead to a proportional increase in reaction rate.
Presence of Inhibitors: Inhibitors are molecules that can bind to enzymes and reduce their activity. Competitive inhibitors block the active site by mimicking the substrate, whereas non-competitive inhibitors attach elsewhere on the enzyme, altering its shape and reducing its activity. Inhibitors can regulate enzyme activity by blocking or reducing catalysis.
Coenzymes and Cofactors: Some enzymes require the presence of additional molecules called coenzymes or cofactors to function properly. Coenzymes are organic molecules, such as vitamins, that assist in enzyme activity. Cofactors are inorganic ions like zinc or magnesium that are vital for enzymes to function properly.
Here are some key functions of enzymes:
Catalysis: The primary function of enzymes is to catalyze or accelerate biochemical reactions by lowering the activation energy required for the reaction to occur. Enzymes enable reactions to proceed at biologically relevant rates, allowing essential processes like metabolism, synthesis, and breakdown of molecules to take place efficiently.
Specificity: Enzymes exhibit remarkable specificity, recognizing and binding to specific substrates. Each enzyme is designed to interact with a particular substrate or group of substrates due to the specific shape and chemical properties of its active site. This specificity ensures that enzymes catalyze specific reactions and do not interfere with unrelated processes.
Regulation: Enzyme activity is often regulated to maintain optimal function and control metabolic pathways. Regulation can occur through feedback inhibition, where the end product of a pathway inhibits an enzyme earlier in the pathway, preventing excessive product formation. Enzyme activity can also be modulated through allosteric regulation, covalent modification, or changes in gene expression.
Metabolism: Enzymes are integral to metabolic pathways involved in energy production, nutrient metabolism, and synthesis of essential molecules. They facilitate processes such as glycolysis, the citric acid cycle, and oxidative phosphorylation, which are vital for converting nutrients into usable energy (ATP) and generating building blocks for cellular components.
Digestion: During digestion, enzymes play an essential role. Various enzymes are involved in breaking down carbohydrates (amylase), proteins (proteases), and lipids (lipases) into smaller, absorbable molecules. These enzymes, produced by the digestive system, help break down food and release nutrients essential for energy and development.
DNA Replication and Transcription: Enzymes like DNA polymerase and RNA polymerase are involved in the replication and transcription of genetic material, respectively. DNA polymerase facilitates the synthesis of new DNA strands during DNA replication, ensuring accurate duplication of the genetic code. RNA polymerase transcribes DNA into RNA molecules, which serve as templates for protein synthesis.