The Arrhenius equation is a fundamental formula in chemical kinetics that explains how the rate of a chemical reaction depends on temperature and activation energy. Named after the Swedish scientist Svante Arrhenius, it quantifies the relationship between the rate constant and temperature.
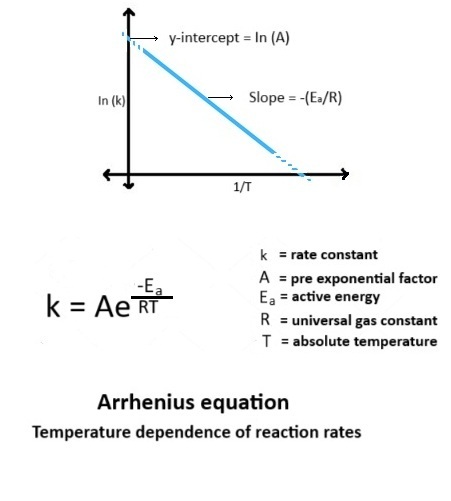
The general form of the Arrhenius equation is:
k = A · e–Ea/RT
Where:
The Arrhenius equation shows that as temperature increases, the exponential term becomes less negative, causing the rate constant k to increase. This means reactions occur faster at higher temperatures because more molecules have sufficient energy to overcome the activation energy barrier.
The Arrhenius equation can be transformed into a linear form by taking the natural logarithm of both sides:
ln k = ln A – Ea/R · (1/T)
This equation is of the form y = mx + c, where:
Plotting ln k against 1/T yields a straight line with a negative slope, which can be used to calculate the activation energy.
The Arrhenius equation provides a clear link between temperature and reaction rate. Its graphical form is a valuable tool for calculating activation energy and understanding the temperature dependence of chemical reactions.